Toward efficient electrocatalytic oxygen evolution with a low concentration baking soda activated IrOx surface in a hydrothermal medium†
Abstract
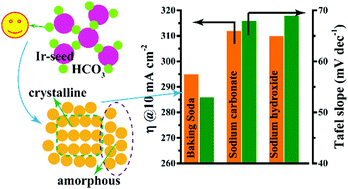
Developing Ir-based catalysts with robust OER performance in a simple, green, and efficient way is critical for scaling up PEM electrolysis to megawatt levels. Here, we report that a low concentration of baking soda significantly increases the OER activity of IrOx nanoparticles and also achieves a yield of 100%. Specifically, the prepared amorphous IrOx affords 10 mA cm−2 with an overpotential of only 295 mV, which is even better than previously reported modified IrO2. Moreover, a Sabatier-like relationship between the work function and the OER activity was clearly revealed and, combined with DFT calculations, the t2g orbitals in IrOx may play a more important role in enhancing the OER activity and they also can be regulated in the amorphous structure via creating more edge-shared IrO6 octahedrons. Atomic-resolution TEM shows that the presence of HCO3− enables the surface atomic arrangement of amorphous IrOx to possess a pseudo-crystalline nature within a short range, which is responsible for the special changes in the orbital structure. According to the extensive experimental results, we believe that HCO3− will likely play an important role in the preparation of Ir-based catalysts with robust OER performance, as baking soda outperforms conventional NaOH in terms of its activity, stability, and green nature.


 Please wait while we load your content...
Please wait while we load your content...