A recyclable covalent triazine framework-supported iridium(iii) terpyridine complex for the acceptorless dehydrogenative coupling of o-aminobenzyl alcohols with ketones to form quinolines†
Abstract
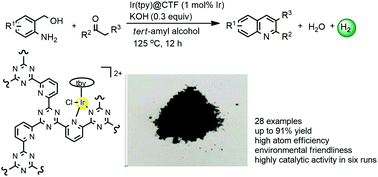
An iridium(III) terpyridine complex Ir(tpy)@CTF, which is designed and synthesized by the coordinative immobilization of [Ir(tpy)Cl3] on a functionalized covalent triazine framework, was proven to be a highly effective catalyst for the acceptorless dehydrogenative coupling of o-aminobenzyl alcohols with ketones. In the presence of the catalyst (1 mol% Ir), a range of desirable products were obtained in high yields. Moreover, the catalyst could be recycled without obvious loss of activity during six runs. Notably, this work exhibited the potential of covalent triazine framework-supported transition metal catalysts for acceptorless dehydrogenation.


 Please wait while we load your content...
Please wait while we load your content...