Efficient and controlled H2 release from sodium formate†
Abstract
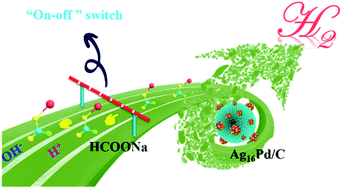
Sodium formate (SF) has been used for a long time as a technological additive for H2 release in the dehydrogenation of formic acid. Formic acid is often synthesized from concentrated sulfuric acid and anhydrous sodium formate at low temperatures, followed by distillation. Therefore, the exploration of direct H2 production by SF decomposition is a practical means of significant importance. Herein, a simple and surfactant-free method was successfully employed to construct M16Pd1/C bimetallic nanomaterials for direct H2 production by SF decomposition in the presence of acetic acid. In particular, the optimal nanocatalyst Ag16Pd1/C showed the highest activity for SF decomposition, with 76.5% H2 yield, 100% H2 selectivity and a TOF of 753 h−1 at 60 °C, whereas no hydrogen was produced from pure sodium formate solution without acetic acid under the same conditions. Interestingly, a highly selective “on–off” switch for on-demand H2 release from SF was successfully developed by pH regulation.


 Please wait while we load your content...
Please wait while we load your content...