Cycloaddition of di-substituted epoxides and CO2 under ambient conditions catalysed by rare-earth poly(phenolate) complexes†
Abstract
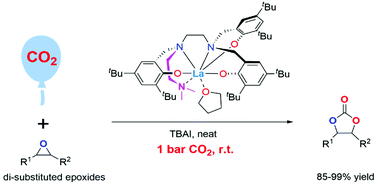
A series of rare-earth (RE) metal complexes [RE = La (1, 5, 6), Nd (2), Sm (3), Y (4)] bearing polydentate N’-(2-aminoethyl)-N,N-dimethyl-1,2-ethylenediamine bridged-tris(phenolto) ligands were synthesized and characterized. The activity of complexes 1–6 for catalysing the cycloaddition reaction of CO2 and epoxides was studied, and the lanthanum complex 1 showed the highest activity. Under the conditions of 1 bar CO2 and 25 °C, lanthanum complex 1 catalysed the reaction of 18 mono-substituted epoxides, generating cyclic carbonates in 50–99% yields. More importantly, it also catalysed reactions of 6 di-substituted epoxides under ambient conditions (1 bar CO2 and 25 °C), and generated cyclic carbonates in 85–99% yields. This lanthanum complex is the first catalyst capable of converting di-substituted epoxides into internal cyclic carbonates under ambient conditions. Kinetic study was conducted to determine rate orders and Gibbs activation energy. A DFT study proved that poly(phenolto) ligands play vital roles in product dissociation.


 Please wait while we load your content...
Please wait while we load your content...