A cyclic thioketone as biradical heterocyclopentane-1,3-diyl: synthesis, structure and activation chemistry†‡
Abstract
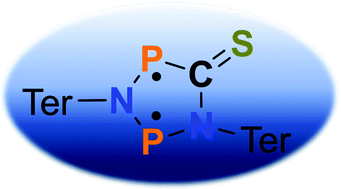
The reaction of the biradical [(μ-NTer)P˙]2 with thiophosgene, SCCl2, leads to a cyclic phospha-aza thiourea derivative in very good yields. This synthetic approach represents a new possibility to prepare cyclic thioketones starting from four-membered cyclo-diphosphadiazanediyls by formal CS insertion with simultaneous oxidation of the two phosphorus atoms by the two chlorine atoms. When the phospha-aza thiourea derivative is reduced with magnesium chips, a deep blue, highly labile cyclic thioketone is formed, which can be regarded as a biradical heterocyclopentane-1,3-diyl. This new biradical can be converted to a housane species by light, which triggers transannular P–P bond formation. A thermal back-reaction was not observed, but scavenging reactions with CS2 or PhCHO clearly indicate the intermediate formation of the labile, biradical cyclic thioketone. Various intercepted products, such as [2.2.1]bicyclic cage compounds could be isolated and structurally characterized. Freshly in situ generated biradical thioketone can be utilized to activate small molecules.


 Please wait while we load your content...
Please wait while we load your content...