Nickel ferrocyanides for aqueous ammonium ion batteries†
Abstract
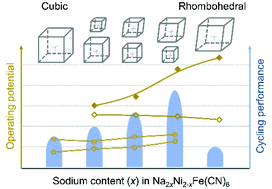
In this report, we design a structural optimization method for Ni2Fe(CN)6 through a partial substitution of nickel with sodium, and investigate the electrochemical performance of a series of Na2xNi2−xFe(CN)6 (x = 0, 0.25, 0.5, 0.75, and 1) materials. During the replacement of nickel with sodium, the structure has turned from a cubic phase to a rhombohedral phase, which results in the changes of electrochemical performance. The electrochemical results reveal that a partial substitution of nickel with sodium can enhance the operating potential, and Na1.5Ni1.25Fe(CN)6 has a better cycling performance than the other four samples. It can provide a reversible capacity of 56.1 mA h g−1 with 93.2% capacity retention after 150 cycles. Interestingly, the sodium content in Na1.5Ni1.25Fe(CN)6 hinders its rate performance. Additionally, we investigate its ammonium-storage mechanism by ex situ XRD and FTIR, which suggests that Na1.5Ni1.25Fe(CN)6 undergoes a phase transition when it is soaked into the electrolyte and exhibits good reversibility and a stable structure during cycling.


 Please wait while we load your content...
Please wait while we load your content...