Crystal–amorphous NiO/MoO2 with a high-density interface for hydrogen evolution†
Abstract
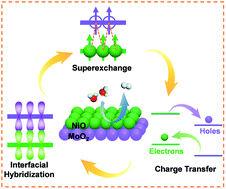
Developing high-density and uniform crystal–amorphous interfaces is highly desirable for the hydrogen evolution reaction (HER). Herein, crystal–amorphous NiO/MoO2 with a coupled high-density interface has been designed to tailor the charge distribution to lower the reaction energy barrier for the HER. The obtained self-supported NiO/MoO2-100-2, which is fabricated through a simple and versatile anodizing-assisted molten salt with a MoNi substrate, possesses high-density and a well-dispersed NiO crystal /amorphous MoO2 heterojunction, benefiting local electron rearrangement and charge transfer. The NiO crystals are beneficial for the alkaline water dissociation to rapidly generate active hydrogen (Volmer step), therefore facilitating the subsequent Heyrovsky and Tafel steps to occur in the amorphous MoO2 region. NiO/MoO2-100-2 exhibits superior HER activity with a low overpotential of 48 mV at 10 mA cm−2, a small Tafel slope of 51.5 mV dec−1 and robust stability, which can be chalked up to the more available actives sites, enhanced conductivity and favorable H adsorption sites derived from the modulated charge and d-band structure near the Fermi level.
- This article is part of the themed collection: FOCUS: Electrocatalytic Hydrogen Evolution


 Please wait while we load your content...
Please wait while we load your content...