Highly red-emissive salen–indium complexes: impact of 4-amino-substitution on the photophysical properties†
Abstract
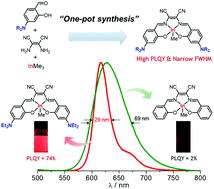
An approach to the design of highly emissive salen–indium complexes is presented. A series of 4-NR2-appended salen ligands possessing an electron-accepting 1,2-dicyanoethylene bridge and their indium complexes (R = Me (1), Et (2), and Ph (3)) were prepared in high yields via a one-pot synthetic pathway. All compounds exhibited narrow-bandwidth red emission (full width at half maximum = 29–42 nm) with high photoluminescence quantum yields (PLQYs, 50%−74%) in toluene. Specifically, 4-NEt2-appended indium complex 2 showed the highest PLQY of 74%, which is among the highest reported for salen-based organometallic luminophores. In contrast, reference complex 4 lacking a 4-amino group was poorly emissive (PLQY < 2%). Theoretical studies suggest that the salen-centered electronic transitions in 1–3 are enhanced by strong electronic interactions between the 4-amino donor and the electron-accepting bridge.


 Please wait while we load your content...
Please wait while we load your content...