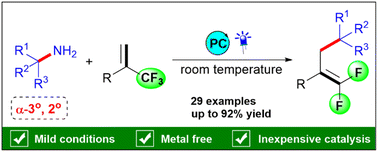
Abstract
Herein, we disclose a new photoredox-catalysed strategy to access gem-difluoroallylarenes from α-trifluoromethylalkenes with sterically hindered primary amines via C–N and C–F bond activation. This deaminative and defluorinative allylation is generally compatible with diverse functional groups and sterically hindered α-3° and 2° primary amines.


 Please wait while we load your content...
Please wait while we load your content...