Cyrene: a bio-based solvent for the Mizoroki–Heck reaction of aryl iodides†
Abstract
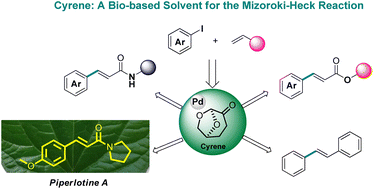
The development of greener and more sustainable methods, as well as the adaptation of already existing protocols to more environmentally friendly procedures, has become crucial for organic synthesis. The introduction and utilization of greener solvents is a very promising alternative, especially when they can replace toxic organic solvents in the known and widely used organic reactions. Cyrene has appeared to be an excellent alternative solvent for a number of organic reactions. In this work, the development of a new, greener and more economical protocol for the Mizoroki–Heck reaction is described, using Cyrene as the green solvent and Pd/C as the palladium catalyst source. A wide substrate scope for the coupling of aryl iodides with acrylamides, acrylates, acrylic acid, acrylonitrile and styrene was demonstrated. The recyclability of Cyrene and the leaching of palladium in the final product were examined in order to enhance the industrial applicability of this protocol. Furthermore, the synthesis of the natural product piperlotine A is reported.


 Please wait while we load your content...
Please wait while we load your content...