Visible-light-initiated external photocatalyst-free synthesis of α,α-difluoro-β-ketoamides from 4-aminocoumarins†
Abstract
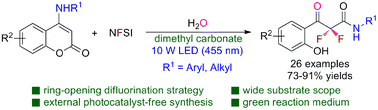
A concise and efficient ring-opening difluorination strategy was developed for the synthesis of highly functionalized hydroxy-containing α,α-difluoro-β-ketoamides from the one-pot multicomponent reaction of 4-aminocoumarins, NFSI, and water in dimethyl carbonate (DMC) as a green solvent. The reactions were smoothly achieved under visible light irradiation in air at room temperature without the addition of any other external photocatalysts. With this protocol, various α,α-difluoro-β-ketoamides were successfully synthesized under mild conditions (25 examples, 73–91% yields). This transition-metal-free synthetic procedure shows good functional group compatibility and attractive practical potential for large-scale synthesis.


 Please wait while we load your content...
Please wait while we load your content...