Redox-neutral rhodium(iii)-catalyzed divergent synthesis of tetrasubstituted 1,3-enynes and alkynylated benzofurans†
Abstract
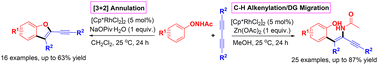
With the assistance of the acetamido directing group (DG), a rhodium-catalyzed C–H alkenylation/DG migration cascade for the synthesis of tetrasubstituted 1,3-enynes from N-phenoxyacetamides and 1,3-diynes has been achieved in this work. Alternatively, a rhodium-catalyzed [3 + 2] annulation for the synthesis of alkynylated benzofurans from the same set of substrates has also been achieved by simply changing the reaction conditions. This work highlights the tunable divergent synthesis of valuable compounds triggered by C–H activation.


 Please wait while we load your content...
Please wait while we load your content...