HFIP-promoted halo-carbocyclizations of N- and O-tethered arene–alkene substrates to access all halo (X = Br, I, Cl)-functionalized tetrahydroquinoline and chroman cores†
Abstract
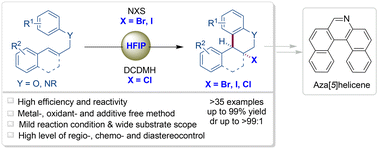
A new method for the stereoselective metal-, additive- and oxidant-free Friedel–Crafts-type halo-carbocyclization of N- and O-tethered arene–olefin substrates is reported, involving reaction with a suitable electrophilic halogenating reagent (NXS/DCDMH) in hexafluoroisopropanol. All halo (X = Br, I, Cl)-functionalized tetrahydroquinolines and chromans were obtained in excellent yields and with high levels of diastereocontrol (dr = >99 : 1), and these products were successfully transformed into synthetically useful compounds such as dihydroquinolines and partial or full azahelicene compounds.


 Please wait while we load your content...
Please wait while we load your content...