An enantio- and diastereoselective approach to indoloquinolizidines in continuous flow†
Abstract
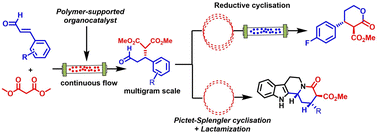
Merging polymer-supported asymmetric organocatalysis with continuous flow in a packed bed reactor has been used as the key, enantiodetermining step in a short synthesis of indoloquinolizidines. Using this approach, a highly enantioselective, solvent-free and rapid conjugate addition of dimethyl malonate to a diverse family of cinnamaldehydes in continuous flow, allowing the preparation of relevant oxodiesters in multigram amounts has been developed. The obtained Michael adducts have been used to complete an expedient diastereoselective synthesis of indoloquinolizidine via cascade Pictet–Splengler cyclisation-lactamisation in continuous flow. The conversion of enantiopure Michael adducts into δ-lactones via telescoped reduction/cyclisation in continuous flow has also been explored.


 Please wait while we load your content...
Please wait while we load your content...