Ligand centered redox enabled sustainable synthesis of triazines and pyrimidines using a zinc-stabilized azo-anion radical catalyst†
Abstract
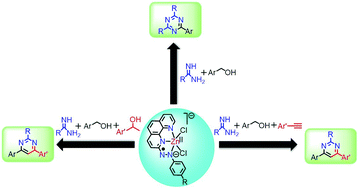
Herein, we report ligand-centered redox controlled Zn(II)-catalyzed multicomponent approaches for synthesizing pyrimidines and triazines. Taking advantage of the ligand-centered redox events and using a well-defined Zn(II)-catalyst (1a) bearing (E)-2-((4-chlorophenyl)diazenyl)-1,10-phenanthroline (L1a) as the redox-active ligand, a wide variety of substituted pyrimidines and triazines were prepared via dehydrogenative alcohol functionalization reactions. Pyrimidines were prepared via two pathways: (i) dehydrogenative coupling of primary and secondary alcohols with amidines and (ii) dehydrogenative coupling of primary alcohols with alkynes and amidines. Triazines were prepared via dehydrogenative coupling of alcohols and amidines. Catalyst 1a is well tolerant to a wide range of substrates yielding the desired pyrimidines and triazines in moderate to good isolated yields. A series of control reactions were performed to predict the plausible mechanism, suggesting that the active participation of the ligand-centered redox events enables the Zn(II)-complex 1a to act as an efficient catalyst for synthesizing these N-heterocycles. Electron transfer processes occur at the azo-aromatic ligand throughout the catalytic reaction, and the Zn(II)-center serves only as a template.


 Please wait while we load your content...
Please wait while we load your content...