Pd-Catalyzed desulfitative arylation of olefins by N-methoxysulfonamide†
Abstract
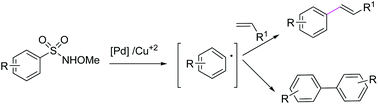
A novel Pd-catalyzed protocol for the desulfitative Heck-type reaction of N-methoxy aryl sulfonamides with alkenes was reported. The cross-coupling reaction was performed successfully with a variety of olefins to obtain aryl alkenes. Different substituents on the aromatic ring of N-methoxysulfonamides were also found to be compatible with the reaction conditions. Expectedly, the reaction proceeds through CuCl2-promoted generation of the nitrogen radical and subsequent desulfonylation under thermal conditions to afford the aryl radical for the Pd-catalyzed coupling reaction. N-Methoxysulfonamide was further exploited for the synthesis of symmetrical biaryls in the presence of CuCl2.


 Please wait while we load your content...
Please wait while we load your content...