Cross dehydrogenation coupling reaction of purine derivatives with thioethers†
Abstract
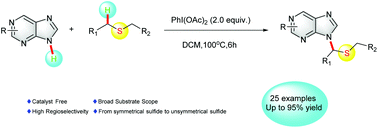
A metal-free cross-dehydrogenation coupling method was established to synthesize N9 alkylated purine derivatives. Using PhI(OAc)2 as the oxidant, versatile thioethers were successfully employed as alkylation reagents. Under the optimized conditions, a variety of alkylated purine derivatives and other aromatic N-heterocycles were obtained in moderate to good yields. The regioselectivity of this protocol which involves the reaction of unsymmetrical thioethers with purine derivatives was also studied.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...