Construction of azaspirocyclic skeletons mediated by the carbonyl of the Weinreb amide: formal total synthesis of (±)-cephalotaxine†
Abstract
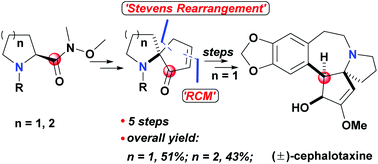
A facile Stevens rearrangement of the Weinreb amide and the subsequent key steps mediated by the carbonyl of the Weinreb amide led to the construction of azaspirocyclic skeletons of some typical alkaloids. And the formal total synthesis of (±)-cephalotaxine was completed via a shorter synthetic route by this efficient method. Further studies on the development and asymmetric synthesis application of this strategy are underway.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...