Synthesis of 2-hydroxytetrahydrofurans by Wacker-type oxidation of 1,1-disubstituted alkenes†
Abstract
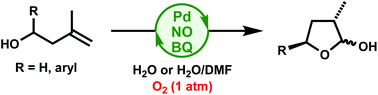
1,1-Disubstituted alkenes feature high steric hindrance, which renders their Wacker-type oxidation difficult. We demonstrate the stereoselective synthesis of 2-hydroxytetrahydrofurans via the Wacker-type oxidation of 3-methyl-3-buten-1-ols by using a PdCl2(MeCN)2/NO/BQ catalyst system under 1 atm O2 in H2O or H2O/DMF.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...