Magnesium dicarboxylates promote the prenylation of phenolics that is extended to the total synthesis of icaritin†
Abstract
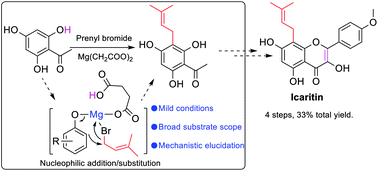
The prenylation of phenolic substrates promoted by magnesium dicarboxylates was developed. An investigation of the scope demonstrated that substrates with electron-donating group(s) gave better yields than those with electron-withdrawing group(s). Although the conversions of all substrates were higher in MeCN than in DMF, DMF was still the favorable solvent for polyphenolic substrates since MeCN would cause the generation of cyclized by-products (6) and reduce the yield of 3. The regio-selectivity of ortho- vs. para-prenylation (3′vs.3′′) for those para-unoccupied substrates was also solvent dependant. DMF produced mainly ortho-products but with poor conversions. On the other hand, MeCN generated mainly para-products, along with minor ortho-products. Mechanistic study of the prenylation provided evidence for the nucleophilic addition/substitution of the phenolic substrate to the alkyl halide in the presence of the magnesium dicarboxylates. The proto application of this method in the total synthesis of icaritin through the prenylation of 2,4,6-trihydroxyacetophenone, followed by the reaction with benzaldehyde to afford the flavonol, was successful, with a total yield of 33%.


 Please wait while we load your content...
Please wait while we load your content...