A squaramide-catalysed asymmetric cascade Michael addition/acyl transfer reaction between unsaturated benzothiophenones and α-nitroketones†
Abstract
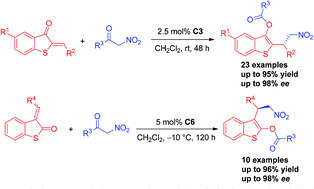
An efficient and practical organocatalytic asymmetric strategy was developed using unsaturated benzothiophenones and α-nitroketones catalysed by bifunctional squaramide via Michael addition and acyl transfer steps. A broad range of chiral acyloxybenzothiophene derivatives were obtained in good yields (up to 97%) with excellent enantioselectivities (up to 98% ee). What's more, employing different chiral squaramide catalysts and unsaturated benzothiophenones can deliver the acyloxy unit at the 2-position or 3-position of benzothiophene.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...