An iron-catalyzed domino reaction of donor–acceptor cyclopropanes: a diastereoselective approach towards diversely functionalized pyrrolo-quinazolines†
Abstract
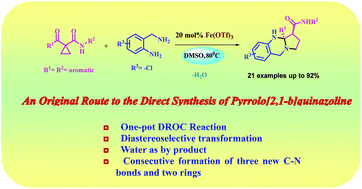
An expeditious synthetic route to access functionalized pyrrolo[2,1-b]quinazoline scaffolds has been achieved via domino ring opening cyclization (DROC) reactions of donor–acceptor (D–A) cyclopropanes and 2-amino(methyl)aniline derivatives. This novel iron catalyzed transformation is amenable to a wide range of substrates. Three new C–N bonds and two rings were sequentially constructed in this divergent one-pot process. The advantages of simple operation, high yields and general applicability make this procedure highly attractive and practical too.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...