Microwave-assisted palladium catalysed C–H acylation with aldehydes: synthesis and diversification of 3-acylthiophenes†
Abstract
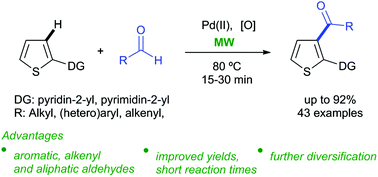
The use of MW allows the efficient palladium(II)-catalysed C-3 acylation of thiophenes with aldehydes via C(sp2)–H activation for the synthesis of (cyclo)alkyl/aryl thienyl ketones (43 examples). Compared to standard thermal conditions, the use of MW reduces the reaction time (15 to 30 min vs. 1 to 3 hours), leading to improved yields of the ketones (up to 92%). The control of positional selectivity is achieved by 2-pyridinyl and 2-pyrimidyl ortho-directing groups at C-2 of the thiophene scaffold. To show the synthetic applicability, selected ketones were subjected to further transformations, including intramolecular reactions to directly embed the directing group in the core structure of the new molecule.


 Please wait while we load your content...
Please wait while we load your content...