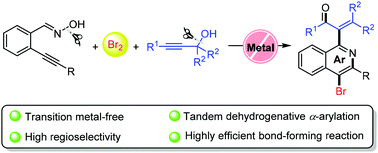
A metal-free tandem dehydrogenative α-arylation reaction of propargylic alcohols with 2-alkynylbenzaldoximes toward the synthesis of α-(4-bromo-isoquinolin-1-yl)-propenone skeletons†
Abstract
A tandem reaction of 2-alkynylbenzaldoximes with propargylic alcohols has been developed for the synthesis of α-(4-bromo-isoquinolin-1-yl)-propenones. Employing 2-alkynylbenzaldoximes as a precursor in the presence of Br2 generates 4-bromo-isoquinoline-N-oxides. Subsequently, dehydroxylation of propargylic alcohols gives carbocation intermediates, which are trapped using the N-oxides, affording aryl-substituted α-enones.


 Please wait while we load your content...
Please wait while we load your content...