Tandem aza-Michael addition–vinylogous aldol condensation: synthesis of N-bridged pyridine fused quinolones†
Abstract
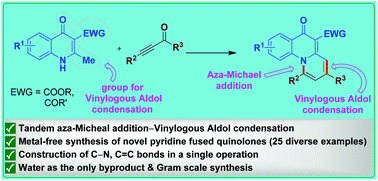
Herein, we present a tandem aza-Michael addition–vinylogous aldol condensation strategy for the synthesis of N-bridged pyridine fused quinolone derivatives from quinolones and ynones. The presented tandem transformation features the construction of C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in a single operation, under transition metal-free conditions. The wide substrate scope and gram scale synthesis of pyridine fused quinolone derivatives expand the synthetic value of the presented protocol.
C bonds in a single operation, under transition metal-free conditions. The wide substrate scope and gram scale synthesis of pyridine fused quinolone derivatives expand the synthetic value of the presented protocol.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...