1,6-Addition of 1,2,3-NH triazoles to para-quinone methides: Facile access to highly selective N1 and N2 substituted triazoles†
Abstract
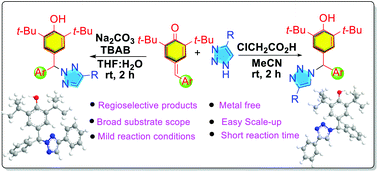
The regioselective syntheses of N1 and N2 substituted triazoles through a 1,6-addition reaction of 1,2,3-NH triazoles to p-quinone methide were achieved under mild reaction conditions. The present reactions showed superior results in terms of selectivity, mild reaction conditions, short reaction time and broad substrate scope with good functional-group compatibility. Considering the high synthetic value of N1- and N2-substituted compounds and p-QM related research, the present strategy will greatly benefit researchers in various fields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...