Optimizing the synergy between alloy and alloy–oxide interface for CO oxidation in bimetallic catalysts†
Abstract
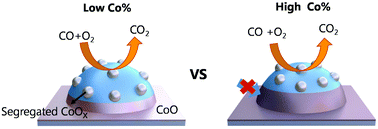
Creating synergetic metal–oxide interfaces is a promising strategy to promote the catalytic performance of heterogeneous catalysts. However, this strategy has been mainly applied to monometallic catalysts, while scarcely applied to alloy catalysts. In this work, we present a comprehensive study on the synergetic alloy–oxide interfaces in the bimetallic Pt–Co/Al2O3 catalysts for CO oxidation. A series of Pt1Cox/Al2O3 catalysts with various Co/Pt molar ratios with x ranging from 0.5 to 3.8 was synthesized via a facile wet-chemistry strategy. Among them, the Pt1Co0.5/Al2O3 catalyst exhibits the best catalytic performance for CO oxidation, with the lowest CO complete conversion temperature of −10 °C and the highest mass specific rate of 2.61 (mol CO) h−1 (g Pt)−1. From in situ X-ray absorption fine structure and diffuse reflectance infrared Fourier-transform spectroscopy studies, the superior catalytic performance of Pt1Co0.5/Al2O3 originates from the optimal length of the three-dimensional alloy-oxide perimeter sites. We further extended this strategy to other bimetallic systems of Pt–Fe and Pt–Ni, which also show similar structural properties and remarkable promotional effects on the catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...