Connecting conformational stiffness of the protein with energy landscape by a single experiment†
Abstract
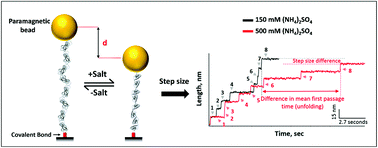
The structure–function dynamics of a protein as a flexible polymer is essential to describe its biological functions. Here, using single-molecule magnetic tweezers, we have studied the effect of ionic strength on the folding mechanics of protein L, and probed its folding-associated physical properties by re-measuring the same protein in a range of ammonium sulfate concentrations from 150 mM to 650 mM. We observed an electrolyte-dependent conformational dynamics and folding landscape of the protein in a single experiment. Salt increases the refolding kinetics, while decreasing the unfolding kinetics under force, which in turn modifies the barrier heights towards the folded state. Additionally, salt enhances the molecular compaction by decreasing the Kuhn length of the protein polymer from 1.18 nm to 0.58 nm, which we have explained by modifying the freely jointed chain model. Finally, we correlated polymer chain physics to the folding dynamics, and thus provided an analytical framework for understanding compaction-induced folding mechanics across a range of ionic strengths from a single experiment.


 Please wait while we load your content...
Please wait while we load your content...