Chemical and morphological characterization of the anodic oxidation of n-GaN in inorganic electrolytes†
Abstract
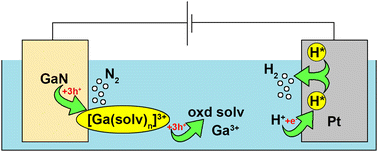
To study the nature of electrochemical property of III-nitrides, we examined here the behaviour of a n-GaN anodic oxidation reaction within a voltage range of 5–20 V in inorganic electrolytes as the pH value varied from 0 to 13. Here, we analysed liquid and vapor phase products of electrochemical oxidation of n-GaN using physicochemical methods and observed different characteristics of the formation of porous n-GaN nanostructures depending on the reaction media. The results show that this reaction is six-electron and proceeds via the formation of surface intermediates. Anodic oxidation of n-GaN forms branching current-oriented nanopores as this process propagates via threading dislocations on the surface of the n-GaN layer.


 Please wait while we load your content...
Please wait while we load your content...