Multifunctional Fe3O4/TiO2/NH2–UiO–66 with integrated interfacial features for favorable phosphate adsorption†
Abstract
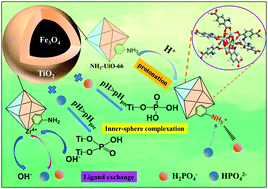
The excessive use and discharge of phosphate are the key factors leading to water eutrophication. Adsorption is deemed to be the most effective method for phosphorus capture. This study prepared a multifunctional synergistic adsorbent (Fe3O4/TiO2/NH2–UiO–66) by the hydrothermal method. The interface assembly of TiO2 and zirconium-based metal–organic frameworks with octahedral-like (NH2–UiO–66) enhanced the adsorption performance of the adsorbent for phosphate and could reach 192.334 mg g−1. The Langmuir adsorption isotherms, pseudo-second-order kinetics, and thermodynamics findings showed that adsorption is monolayer chemisorption and a spontaneous endothermic process. After five cycles of adsorption experiments, the adsorbent could still maintain more than 80% of the initial adsorption capacity. FT-IR spectroscopy, XPS, and DFT analysis proved that the adsorbent was bonded by inner-sphere complexation and ligand exchange mechanisms with phosphate. Under acidic conditions, the amino group was protonated to form an ammonium ion, adsorbed by electrostatic attraction with phosphate. Unlike previous studies, the synergistic action between the interface of TiO2 and NH2–UiO–66 can act as a “claw” for capturing phosphate and exhibits efficient adsorption capacity and pH adaptability for phosphate. It is expected to be well applied to practical water treatment.


 Please wait while we load your content...
Please wait while we load your content...