Steric effects of a homogeneous CuCl2/solvent system for photocatalytic selective oxidation of benzyl alcohol
Abstract
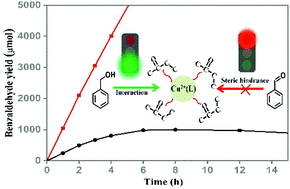
Homogeneous catalytic conversion of benzyl alcohol into benzaldehyde has been widely reported, but the selectivity may be limited due to the overoxidation of benzaldehyde in the presence of benzyl alcohol. Importantly, such overoxidation reaction mechanism in a homogeneous reaction system is still elusive. In this work, the homogeneous CuCl2/solvents system was used as a model system to explore the difference between the ideal system and the homogeneous system for the selective oxidation of benzyl alcohol to benzaldehyde and the autoxidation of benzaldehyde to benzoic acid. It was found that sterically hindered solvents (ligands) cause a switch of the catalytic performance of the CuCl2/solvents photocatalytic system towards oxidation of benzyl alcohol. Within the environment of the copper complex, sterically hindered solvents as scaffolds provide more probability of collision and interaction between the copper complex and benzyl alcohol by blocking the produced benzaldehyde, which gives a high yield of benzaldehyde.


 Please wait while we load your content...
Please wait while we load your content...