Tandem amide coupling and hydroamination: unexpected benzotriazole oxide addition to the propiolic acid triple bond†
Abstract
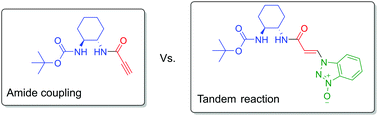
Amides are common molecules which are most often prepared using benzotriazole oxide coupling reagents. Such amides can be derivatised with compounds that contain a triple carbon–carbon bond in order to facilitate easy introduction of the amide fragment into a target molecule via cycloaddition reactions. In this paper, a previously unknown tandem reaction consisting of amide coupling and hydroamination of a triple C–C bond is described. The reaction occurs when the TBTU/HOBt coupling reagent pair is used for coupling of propiolic acid with mono-Boc-diaminocyclohexane. The hydroamination can be circumvented by using COMU as the coupling reagent. The addition reaction was further explored by using methyl propiolate with TBTU/HOBt which yielded a plethora of different products including O- and N-addition products and also a product of formal methyl propiolate dimerization. Due to high symmetry and low number of non-equivalent protons and carbons, most of the products were difficult to characterise by NMR spectroscopy, so it was necessary to obtain X-ray single crystal structures for most of them. In addition, the identification of the products was further supported by very good agreement of NMR spectra collected experimentally with NMR spectra calculated by DFT.


 Please wait while we load your content...
Please wait while we load your content...