MIL-101 supported CeOx-modified NiPt nanoparticles as a highly efficient catalyst toward complete dehydrogenation of hydrazine borane†
Abstract
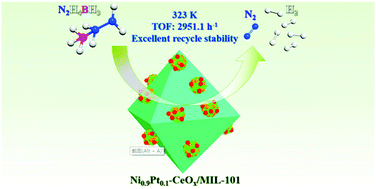
The high hydrogen content and excellent stability at room temperature promote hydrazine borane (HB, N2H4BH3) as a promising chemical hydrogen storage material. However, the development of highly efficient and selective catalysts remains a challenge for the practical use of HB as a hydrogen source. In this work, NiPt–CeOx nanoparticles anchored on MIL-101 support are successfully synthesized by a facile wet impregnation reduction method at room temperature. The as-synthesized Ni0.9Pt0.1–CeOx/MIL-101 catalyst exhibits excellent catalytic activity, 100% conversion rate, 100% hydrogen selectivity and outstanding recycling stability for hydrogen production from HB under alkaline conditions. A high turnover frequency (TOF) value of 2951.1 h−1 is achieved at 323 K even when the content of Ni is up to 90%, which is much higher than most reported non-noble-metal-containing catalysts for HB dehydrogenation. Such excellent performance may be attributed to the well-dispersed ultrafine NiPt–CeOx nanoparticles (3.35 nm) anchored by MIL-101, the unique amorphous/low crystallinity structure and the electron synergistic effect between different metals. Additionally, the presence of NaOH can promote the difficult breaking of the N–H bond during the interaction between HB and the surface of Ni0.9Pt0.1–CeOx, thus further improving the efficiency of HB decomposition. This easily prepared, highly active, and low-cost catalyst can promote the practical application of HB as a potential hydrogen storage material.


 Please wait while we load your content...
Please wait while we load your content...