Tunable Pd/C-catalyzed oxidative alkoxycarbonylation/aminocarbonylation of aryl hydrazines with alcohols/inert tertiary amines through C–N bond activation†
Abstract
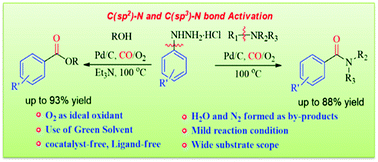
We report Pd/C-catalyzed oxidative aminocarbonylation and alkoxycarbonylation of unactivated aryl hydrazines. This protocol employs inert tertiary amines as an aminal source and arylhydrazines via oxidative sp3 and sp2 C–N bond activation using molecular oxygen as an oxidant. The tertiary amine acts as both a nucleophile and a base. Advantageously, this process involves Pd/C as a heterogeneous and recyclable catalyst, and propylene carbonate as an environmentally benign solvent. This established protocol provides a promising, simple, and effective approach for preparing esters and tertiary amides under co-catalyst-free and moisture sensitive ligand-free conditions. This catalytic process features sustainable and resourceful, recyclable, and valuable products.


 Please wait while we load your content...
Please wait while we load your content...