Novel A–D–A structural imidazole derivatives with charge transfer excited states: importance of molecular structure design in obtaining a “turn-on” type fluorescence probe†
Abstract
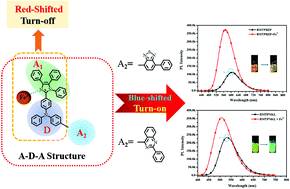
Herein, two novel A–D–A structural imidazole derivatives, namely BMTPBZP and BMTPNKL, with orange and green fluorescence were successfully designed and synthesized, which exhibited an evident charge transfer (CT) excited state property. Compared with the triphenylamine-imidazole molecule TPA-BM with a simple D–A structure, which made the fluorescence red-shifted and quenched the response toward Fe3+, both BMTPBZP and BMTPNKL could selectively recognize Fe3+ based on the infrequent response of fluorescence enhancement and a blue shift with a relatively great sensitivity and anti-interference performance, suggesting that they have potential to be used as a novel fluorescence turn-on type Fe3+ sensor. The sensing mechanism study showed that the response of these two probe molecules toward Fe3+ could be attributed to the coordination reaction between Fe3+ and the electron-rich N atom on the imidazole ring in a 1 : 1 ratio, which caused the CT excited state of the probe molecules to generate a new and higher energy CT emission. In addition, the introduction of the A–D–A structure was demonstrated to be the key for obtaining the enhanced fluorescence and blue-shifted response of the probe molecules.


 Please wait while we load your content...
Please wait while we load your content...