Synthesis, computational and nanoencapsulation studies on eugenol-derived insecticides†
Abstract
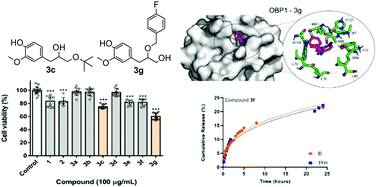
A new set of alkoxy alcohols were synthesised by the reaction of eugenol oxirane with aliphatic and aromatic alcohols. These eugenol derivatives were evaluated against their effect upon the viability of an insect cell line Sf9 (Spodoptera frugiperda). The most promising compounds, 4-(3-(tert-butoxy)-2-hydroxypropyl)-2-methoxyphenol and 4-(2-((4-fluorobenzyl)oxy)-3-hydroxypropyl)-2-methoxyphenol, were submitted to in silico assays to predict possible targets. Using an inverted virtual screening approach, 23 common pesticide targets were screened and the top 2 targets predicted were further analyzed using molecular dynamics simulations and free energy calculations. In addition, these eugenol derivatives were subjected to encapsulation and release assays using liposome-based nanosystems of egg phosphatidylcholine : cholesterol (7 : 3), with encapsulation efficiencies of higher than 90% and release profiles well described by both Korsmeyer–Peppas and Weibull models.


 Please wait while we load your content...
Please wait while we load your content...