Bimetallic PdII complexes with NHC/Py/PCy3 donor set ligands: applications in α-arylation, Suzuki–Miyaura and Sonogashira coupling reactions†
Abstract
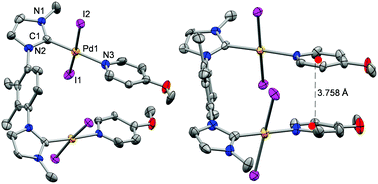
Bimetallic complexes bearing NHC donor ligands are gaining immense popularity as catalysts in organometallic chemistry. However, such complexes with a mixed NHC/PR3 donor set ligands are still rare. We present here a series of bimetallic PdII complexes featuring mixed NHC/Py/PCy3 donor set ligands applied in α-arylation of amide, Suzuki–Miyaura coupling and Sonogashira coupling reactions. All complexes have been characterized using standard characterization techniques and one of the complexes has been structurally characterized by X-ray crystallographic analysis. Catalytic outcomes clearly indicate the superior activity of PdII complex bearing mixed NHC/PCy3 donor set ligands compared to the PEPPSI-type complexes in both α-arylation of amide and Sonogashira coupling reactions, though almost similar activities were observed for all the complexes in Suzuki–Miyaura coupling reactions. This observation might be due to the involvement of Pd–NPs as an active catalyst in Suzuki–Miyaura coupling reactions, whereas the Hg-poison test suggested the homogeneous nature of the other two types of reactions. Two pyridine rings in a mixed NHC/4-OMe(Py) donor complex are oriented almost in a parallel face-to-face fashion with a centroid to centroid separation of 3.758 Å, indicating π···π interactions between two methoxypyridine rings. This interaction leads to a very short Pd···Pd distance of 5.754 Å in the aforementioned complex.


 Please wait while we load your content...
Please wait while we load your content...