Synthesis of new chiral N-heterocyclic diselenides and their application in the alkoxyselenylation reaction†
Abstract
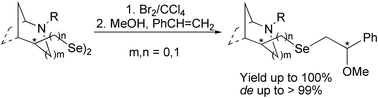
This paper describes the synthesis of chiral diselenides based on 2-azabicycloalkane or the pyrrolidine skeleton using in situ generated Na2Se2 as an efficient selenylating reagent. All the diselenides were obtained in moderate yields under mild conditions. The well-defined enantio- and diastereomerically pure compounds were tested in the asymmetric alkoxyselenylation of alkenes yielding products with diastereoisomeric excess up to >99%.
- This article is part of the themed collection: 50th anniversary of ICCST: celebrating ICCST at its 15th Edition


 Please wait while we load your content...
Please wait while we load your content...