Copper and neocuproine catalysed synthesis of cinnamyl ether derivatives directly from secondary and tertiary cinnamyl alcohols†
Abstract
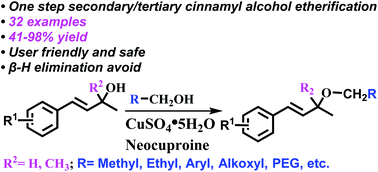
The skeletons of secondary and tertiary cinnamyl alcohols are found ubiquitously in natural products and commercial drugs. Due to the easy dehydration of their structures, the etherification of such compounds is often difficult. A new method for the synthesis of secondary and tertiary cinnamyl ether derivatives, employing copper sulfate pentahydrate (CuSO4·5H2O) as a catalyst and neocuproine as a ligand, was developed. Interestingly, this method enabled the efficient connection of the long alkoxy chain with secondary or tertiary cinnamyl alcohols. Through this method, PEG-modified secondary and tertiary cinnamyl alcohols containing natural products and small molecule drugs were realized, providing a green method to generate functional cinnamyl ether derivatives directly.


 Please wait while we load your content...
Please wait while we load your content...