Photo-mediated synthesis of 1,2-dideoxy-2-phosphinylated carbohydrates from glycals: the reduction of glycosyl radicals to glycosyl anions†
Abstract
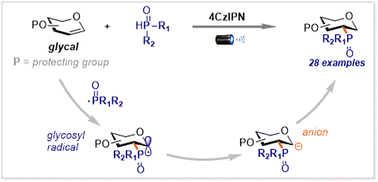
We have developed a photo-mediated method for the synthesis of C2-phosphinylated carbohydrates from glycals and diarylphosphine oxides. This approach avoids over-stoichiometric transition-metal promoters used in previous methods and shows a broader substrate scope in terms of both glycals and diarylphosphine oxides, which is well manifested by substrates with one or even three hydroxy groups. Furthermore, the reaction is also suitable for the late-stage modification of complex molecules and for the introduction of the phosphorous group to the 6-position of carbohydrates. The reaction pathway includes a sequence of the generation of a glycosyl radical by the addition of a phosphoryl radical to a glycal substrate, and the reduction of the glycosyl radical to a glycosyl anion. The latter approach is verified by the labeling experiments and DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...