One-pot chemoenzymatic synthesis of glycolic acid from formaldehyde†
Abstract
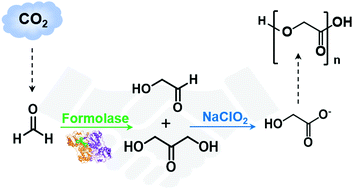
Glycolic acid is an important building block of biodegradable polymers. Herein, we report an efficient selective and atom-economical approach for the synthesis of glycolic acid based on a catalytic C–C coupling reaction of the inexpensive C1 compound formaldehyde followed by selective oxidation. The developed approach relies on an evolved formolase with improved activity at a high formaldehyde concentration (up to 1 M) and controlled oxidation by the oxidant, sodium chlorite or H2O2. Kinetic characterization, molecular dynamics simulation and binding free energy calculation demonstrated that the identified amino acid substitutions of the evolved formolase stabilize the first reaction intermediate state TPP-FA (thiamine pyrophosphate-formaldehyde) and the second intermediate state TPP-GA (thiamine pyrophosphate-glycolaldehyde), which is beneficial for the C–C coupling product generation, especially C2 GA at a high concentration of formaldehyde. The chemoenzymatic approach established in this work provides a new opportunity to manufacture monomers of biodegradable polymers from CO2 derivatives.


 Please wait while we load your content...
Please wait while we load your content...