Near quantitative conversion of xylose into bisfuran†
Abstract
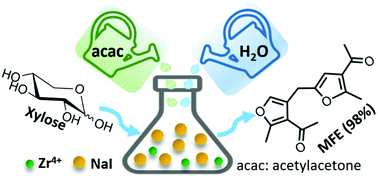
Renewable and abundant carbohydrates are promising feedstocks for producing valuable chemicals. Here we report a highly efficient Zr-catalysed conversion of xylose and acetylacetone (acac) to a new type of bisfuranic monomer, 1-(4-((4-acetyl-5-methylfuran-2-yl)methyl)-2-methylfuran-3-yl)ethenone (MFE). The formation of MFE stems from the intermediate obtained through the nucleophilic addition of acac to xylose. Under optimized conditions (microwave irradiation, 140 °C, 24 min, NaI as an additive), MFE is obtained in near-quantitative yield (98%). Importantly, the reaction selectivity can be tuned by the inclusion of an additive. When NaCl is used, the reaction gives 3-(furan-2-ylmethylene)pentane-2,4-dione (FMPD, 55%), a jet-fuel precursor, and MFE (30%) with a total carbon yield of 85%. To the best of our knowledge, this is the first report on straightforward xylose transformation to a bisfuranic compound with excellent carbon efficiency. This Garcia Gonzalez (GG) reaction inclusive strategy is remarkable and could lead to many innovations in bio-based polymer synthesis.
- This article is part of the themed collection: 2022 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...