New electrotriggers: p-methoxycarbonylbenzyl (pMCB) as an electroremovable protecting group for carboxylic acids, phosphoric acids and alcohols†
Abstract
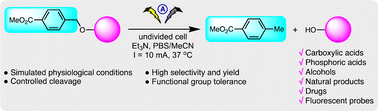
Precise release of bioactive molecules is highly desirable in order to optimise drug therapy. Organic electrochemistry offers a highly versatile tool for the selective cleavage of chemical bonds using electrons as traceless reagents. We present herein an electrotriggered reaction that can specifically cleave C–O bonds in response to electricity under simulated physiological pH conditions. Using this strategy, we show that carboxylic acids including natural products, drugs and fluorescent probes, as well as phosphoric acids and alcohols, are released in a controllable manner. In addition, a water-soluble leaving group based on the p-methoxycarbonylbenzyl moiety is proposed as an electrocleavable linker. We envision that this on-demand C–O cleavage will provide unique opportunities in biological research and therapeutics.


 Please wait while we load your content...
Please wait while we load your content...