Oxidative cleavage of C–C double bond in cinnamic acids with hydrogen peroxide catalysed by vanadium(v) oxide†
Abstract
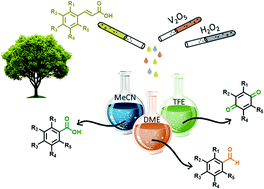
We have developed a cheap, green, mild and environmentally friendly method for the selective cleavage of carbon–carbon double bonds with a 30% aqueous solution of hydrogen peroxide as the oxidant and vanadium(V) oxide as the catalyst. The selectivity of the oxidative cleavage of cinnamic acid derivatives 1 depends on the substituents and the solvent used (DME – MeOCH2CH2OMe, TFE – 2,2,2-trifluoroethanol or MeCN). In DME, p-hydroxy derivatives were selectively converted to benzaldehyde derivatives 2, in TFE, oxidative cleavage led to the formation of benzoquinone derivatives 4, while in MeCN, cinnamic acid derivatives were selectively converted to benzoic acid derivatives 3. Ferulic acid 1a was quantitatively and selectively converted to vanillin 2a in a 91% isolated yield on a gram scale. Dimeric difurandione 1a′ was isolated as an intermediate, which was confirmed by in situ ATR-IR spectroscopy, while the formation of diols or epoxides was not observed. The analogous styrene derivative, 4-vinylguaiacol 1e was also selectively converted to either vanillin 2a or 2-methoxyquinone 4a in a high yield. The green metric for the conversion of ferulic acid to vanillin by different methods was calculated and compared to our method, and showed that our method has better environmental parameters.


 Please wait while we load your content...
Please wait while we load your content...