Effect of sodium silicate on drinking water biofilm development†
Abstract
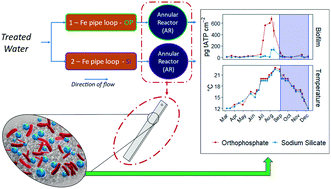
Sodium silicates have been studied as drinking water additives for coagulation, sequestration of iron, and corrosion control, but their impact on biofilm formation has received less attention. This study investigated the impact of sodium silicate corrosion control on biomass accumulation in comparison to orthophosphate, a common corrosion inhibitor. Biofilm growth was measured by determining ATP concentrations, and bacterial communities were characterized using 16S ribosomal RNA (rRNA) sequencing. In a pilot-scale study with annular reactors (ARs) fed by cast iron pipe loops, biofilm ATP concentrations were substantially lower on polycarbonate coupons in the sodium silicate-treated AR than on those in the orthophosphate-treated AR when the water temperature exceeded 20 °C. An elevated sodium silicate dose (48 mg L−1 of SiO2), however, dispersed the biofilm, resulting in elevated effluent ATP concentrations. Two separate experiments confirmed that biomass accumulation was higher in the presence of orthophosphate at high water temperatures (≥20 °C), whereas no significant differences were identified in biofilm ATP concentrations at lower water temperatures (<20 °C). Differences in bacterial communities between the orthophosphate- and sodium silicate-treated systems were not statistically significant, even though orthophosphate promoted higher biofilm growth. The genera Halomonas and Mycobacterium, however—which include opportunistic pathogens—were present at greater relative abundances in the orthophosphate- compared to the silicate-treated system.
- This article is part of the themed collection: Recent Open Access Articles


 Please wait while we load your content...
Please wait while we load your content...