Near-unity electrochemical conversion of nitrate to ammonia on crystalline nickel porphyrin-based covalent organic frameworks†
Abstract
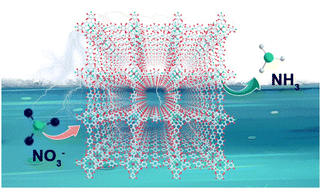
Electrochemical nitrate reduction, which has attracted rapidly increasing attention over recent years, can potentially enable the indirect fixation of atmospheric N2 as well as the efficient removal of nitrate from industrial wastewater. It is, however, limited by the lack of efficient and low-cost electrocatalysts available so far. To address this challenge, we here demonstrate a two-dimensional nickel porphyrin-based covalent organic framework (COF) as a potential candidate for the first time. The product has a highly ordered molecular structure with abundant square-shaped nanopores. In neutral solution, the reduction of nitrate ions at different concentrations from ammonia is realized with a great selectivity of ∼90% under a mild overpotential, a remarkable production rate of up to 2.5 mg h−1 cm−2, a turnover frequency of up to 3.5 s−1, and an intrinsic stability that is best delivered under pulse electrolysis. This cathodic reaction can also be coupled with the oxygen evolution reaction to enable full-cell electrolysis at high efficiency. Theoretical computations indicate that nickel centers can stably adsorb nitrate, and facilitate its subsequent reduction by lowering the energy barrier of the rate-determining step.


 Please wait while we load your content...
Please wait while we load your content...