A self-purifying electrolyte enables high energy Li ion batteries†
Abstract
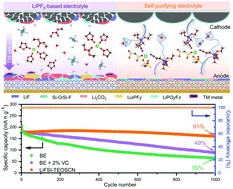
Conventional LiPF6/carbonate electrolytes with poor oxidative stability and reactive decomposition products (HF, PF5, POF3, etc.) dictate less-stable electrode/electrolyte interphases, which thereby promote the dissolution of transition metal ions, accelerate the constant decomposition of electrolyte solvent, and result in the degradation of LIBs. Herein, we demonstrate a new type of electrolyte, id est, 1.6 M lithium bis(fluorosulfonyl)imide (LiFSI) in (2-cyanoethyl)triethoxysilane (TEOSCN) with a self-purifying feature. TEOSCN molecules in the electrolyte can effectively eliminate the reactive pernicious species, while the anions of FSI− dominate the interphase components with low-resistance on both graphite and Ni-rich NMC cathode although at an essentially low concentration. This self-purifying electrolyte system enables long-term cycling of MCMB‖NMC811 full-cells for 1000 cycles with an ultra-high capacity retention of 91% at 25 °C and for 500 cycles with a retention of 81% at 60 °C. Even in extreme cases, i.e., exposed in the air for 1 h, this electrolyte still allows the stable charge–discharge cycling of MCMB‖NMC811 full-cells without degradation, which can largely simplify the manufacturing processes of LIBs. The ‘self-purifying-plus-passivation’ strategy opens a promising frontier for electrolyte engineering towards next-generation high-energy LIBs.


 Please wait while we load your content...
Please wait while we load your content...