The effect of alkyl substitution position of thienyl outer side chains on photovoltaic performance of A–DA′D–A type acceptors†
Abstract
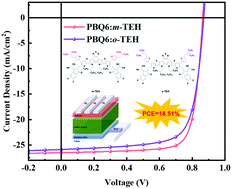
The side chain configuration has shown significant influence on the intermolecular interaction and aggregation morphology of organic small molecule acceptors (SMAs). In this work, we designed and synthesized two isomeric A–DA′D–A type SMAs with thienyl outer side chains attaching a 2-ethylhexyl substituent at the α- or β-position (named o-TEH and m-TEH respectively) for investigating the effect of thienyl conjugated outer side chains and the alkyl substitution position of thienyl outer side chains on the properties of SMAs. Compared to o-TEH with α-substitution, β-substituted m-TEH shows closer π–π stacking, stronger intermolecular interaction and higher electron mobility. In addition, the blend films of m-TEH with the PBQ6 polymer donor possess more suitable phase separation, enhanced molecular packing, higher and more balanced hole and electron mobilities, longer charge carrier lifetime and less charge carrier recombination than PBQ6:o-TEH based blend films. Organic solar cells (OSCs) based on PBQ6:m-TEH achieved a power conversion efficiency (PCE) of 18.51%, which is significantly higher than the PCE (16.22%) of PBQ6:o-TEH based OSCs. Up to now, 18.51% is one of the highest PCEs reported for binary OSCs, indicating that m-TEH with 2-ethylhexyl β-substituted thienyl outer side chains is an excellent high-performance SMA for OSCs.


 Please wait while we load your content...
Please wait while we load your content...