The low overpotential regime of acidic water oxidation part II: trends in metal and oxygen stability numbers†
Abstract
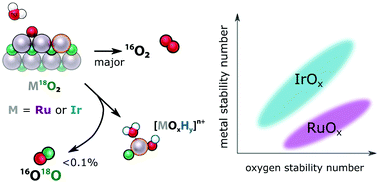
The operating conditions of low pH and high potential at the anodes of polymer electrolyte membrane electrolysers restrict the choice of catalysts for the oxygen evolution reaction (OER) to oxides based on the rare metals iridium or ruthenium. In this work, we investigate the stability of both the metal atoms and, by quantitative and highly sensitive 18O isotope labelling experiments, the oxygen atoms in a series of RuOx and IrOx electrocatalysts during the OER in the mechanistically interesting low overpotential regime. We show that materials based on RuOx have a higher dissolution rate than the rate of incorporation of labelled oxygen from the catalyst into the O2 evolved (“labelled OER”), while for IrOx-based catalysts the two rates are comparable. On amorphous RuOx, metal dissolution and labelled OER are found to have distinct Tafel slopes. These observations together lead us to a full mechanistic picture in which dissolution and labelled OER are side processes to the main electrocatalytic cycle. We emphasize the importance of quantitative analysis and point out that since less than 0.2% of evolved oxygen contains an oxygen atom originating from the catalyst itself, lattice oxygen evolution is at most a negligible contribution to overall OER activity for RuOx and IrOx in acidic electrolyte.
- This article is part of the themed collection: Recent Open Access Articles


 Please wait while we load your content...
Please wait while we load your content...