Interphase control for high performance lithium metal batteries using ether aided ionic liquid electrolyte†
Abstract
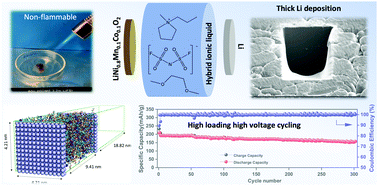
Future rechargeable Li metal batteries (LMBs) require a rational electrolyte design to stabilize the interfaces between the electrolyte and both the lithium metal anode and the high voltage cathode. This remains the greatest challenge in achieving high cycling performance in LMBs. We report an ether-aided ionic liquid electrolyte which offers superior Li metal deposition, high voltage (5 V) stability and non-flammability. High performance cycling of LiNi0.8Mn0.1Co0.1O2 (4.4 V) and LiNi0.6Mn0.2Co0.2O2 (4.3 V) cells is demonstrated with high coulombic efficiency (>99.5%) at room temperature and elevated temperatures, even at high practical areal capacity for the latter of 3.8 mA h cm−2 and with a capacity retention of 91% after 100 cycles. The ether-ionic liquid chemistry enables desirable plated Li microstructures with high packing density, minimal ‘dead’ or inactive lithium formation and dendrite-free long-term cycling. Along with XPS studies of cycled electrode surfaces, we use molecular dynamics simulations to demonstrate that changes to the electrolyte interfacial chemistry upon addition of DME plays a decisive role in the formation of a compact stable SEI.


 Please wait while we load your content...
Please wait while we load your content...
